Based on Equilibrium Considerations Which Adsorbent Is Best
Mg-MOF-74 is predicted as the most favourable by four metrics while Zeolite 13X and UTSA. 2 points Which component is most strongly adsorbed by each adsorbent.
Adsorbent and adsorbate.

. Since the modeled system involves ion adsorption the more appropriate reference state for Boltzmann distribution is the ion equilibrium concentration in the bulk fluid phase CE. Charcoal silica gel alumina gel are good adsorbents because they have highly porous structures and have large surface area. Adsorption isotherms show the relationship between partial pressure of a gas molecule and its equilibrium loading on the adsorbent material at a.
Adsorbent coatings for heat pumping applications. Efficient than under quasi-equilibrium conditions. Verification of hydrothermal and mechanical stabilities By Lucio Bonaccorsi and L.
2 points Which adsorbent has the greatest selectivity. Typically adsorber design entails use of the following methodology. Adsorbent ZMS 4A ZMS 5A 3 Ideal or Best Adsorbent The separation of propane Cs and propylene C3 is accomplished by distillation but at the expense of more than 100 trays and a reflux ratio greater than 10.
All adsorbent metrics consistently ranked CS-AC as the least favourable. If the solid and fluid are placed in contact for a long time an equilibrium distribution is reached and. Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper.
2 points Based on equilibrium considerations which adsorbent is best. Pore size the strength of adsorbate-adsorbent interactions and adsorbent heterogeneity. 5 C C E e μ q V k B T where μ is the chemical.
However there is no clear winner predicted by the adsorbent metrics. The substance on the surface of which adsorption occurs is known as adsorbent. Consequently the use of adsorption has been investigated in a number of studies.
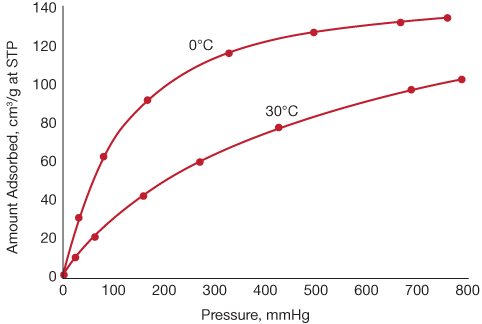
From an equilibrium based MOF adsorbent to a kinetic selective carbon molecular sieve for paraffiniso-paraffin separation Chem Commun Camb. The substances that get adsorbed on the solid surface due to intermolecular attractions are called adsorbate. A typical equilibrium isotherm is shown in Figure 2.
Adsorbent bed profile and media loading capacity characteristics for the specific application and adsorbent material used. -m b and t are constants for a given adsorbate-adsorbent and T-Obeys Henrys law at low P and reaches a maximum at high P-Reduce to the Langmuir isotherm for t 1 UNILAN isotherm ln 2 s s n c pe q s c pe-é ù ê ú ë û-n s and c are constants for a given adsorbate-adsorbent and T-Based on a model of heterogeneous surfaces. Most PSA systems are based on equilibrium considerations.
Pressure drop characteristics across the adsorbent bed. Calabrese Water adsorption on zeolite 13X. Thermodynamic requirements to an optimal adsorbent which ensures the best cycle performance under prescribed operating conditions were reported for non-regenerative AHT cycles in Aristov 2005.
Comparison of the two methods based on mass spectrometry and thermogravimetry. Kinetics and equilibrium studies J Hazard Mater. Curves was obtained with the best fit corresponding to the.
Authors Baiyan Li 1. Intelligent choice of adsorbent should be based on comprehensive analysis that takes into consideration both thermodynamic and dynamic aspects. 2 points Which adsorbent has the greatest capacity.
Adsorbent should be based on comprehensive analysis that. Equilibrium considerations The adsorption process can be considered a partitioning of the adsorbate between the fluid phase and the adsorbent. This increases the adsorp-tion strength leading to more adsorption at lower pressures.
Fixed-bed adsorber design is based upon the following considerations. An adsorbents pore size affects the equilibrium uptake as narrow pores result in greater overlap of the adsorption potentials of opposing pore walls.

Solved Adsorbent Zms 4a Zms 5a 3 Ideal Or Best Adsorbent Chegg Com

Equilibrium Adsorption Isotherms Of A Co 2 B Ch 4 C N 2 Download Scientific Diagram

A Kinetic Equilibrium And Thermodynamic Study Of L Phenylalanine Adsorption Using Activated Carbon Based On Agricultural Waste Date Stones Journal Of Applied Research And Technology Jart

Experimental Equilibrium Data And The Best Fitted Isotherm Model For Download Scientific Diagram

Solved The Separation Of Propane C3 And Propylene Cz Is Chegg Com

Adsorption Equilibrium Isotherm Of Cr Vi Onto Zbac Model L And Model Download Scientific Diagram

Single Component Adsorption Equilibrium On Zeolite 13x For A Co2 And B Download Scientific Diagram

A Adsorption Equilibrium B Langmuir And C Freundlich Isotherm Download Scientific Diagram

A Equilibrium Isotherms Of Oil Adsorption Onto Modified Calcium Download Scientific Diagram

Adjustment Of Kinetic Models A And Adsorption Equilibrium Isotherm Download Scientific Diagram

Acetaminophen Equilibrium Adsorption At Different Temperatures Onto Download Scientific Diagram

Adjustment Of Kinetic Models A And Adsorption Equilibrium Isotherm Download Scientific Diagram
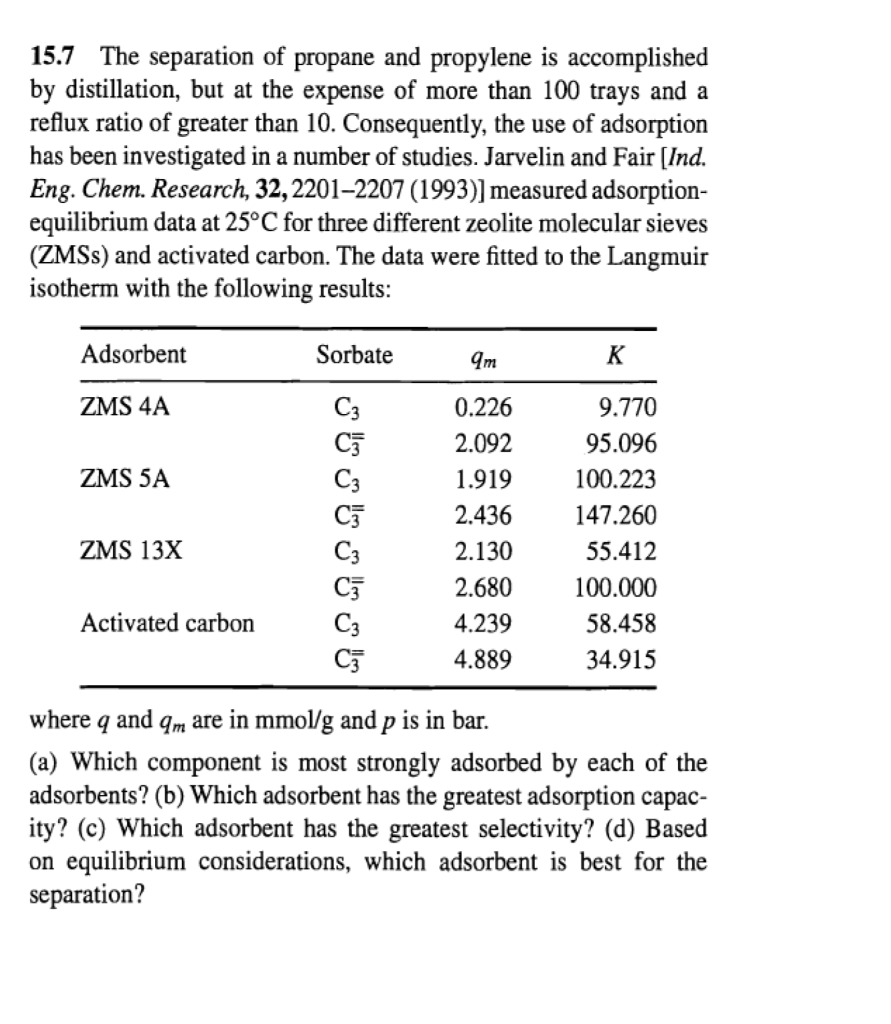
15 7 The Separation Of Propane And Propylene Is Chegg Com

Acetaminophen Equilibrium Adsorption At Different Temperatures Onto Download Scientific Diagram

A Equilibrium Adsorption Isotherm Of Af Dye With Different Initial Download Scientific Diagram

Relationship Between Equilibrium Phenol Concentration And Its Download Scientific Diagram
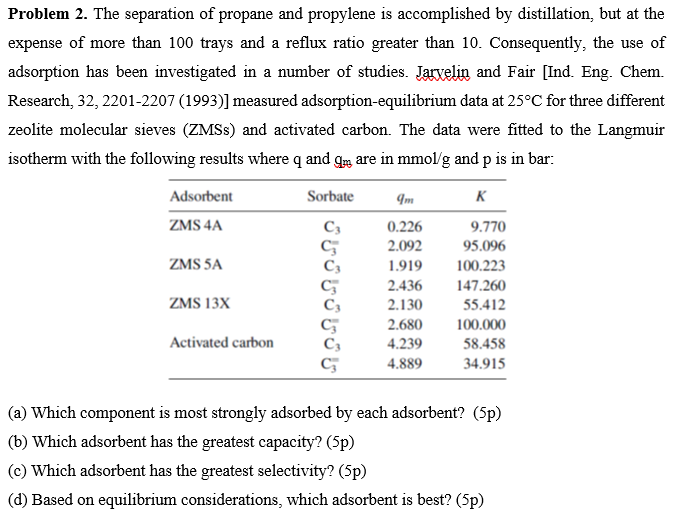
Problem 2 The Separation Of Propane And Propylene Is Chegg Com
Comments
Post a Comment